To provide further clarification on the virus that causes COVID-19 infections, which is technically named SARS-CoV-2, and concerns about how it relates to residuals, sludge, and biosolids for water resource recovery facilities (WRRFs) as well as the wastewater sector at large, this article includes a review of available data related to the virus and surrogates as well as their potential associations with residuals, sludge, and biosolids. This update is intended to supplement to the currently published article, “The Water Professionals Guide to COVID-19” the Waterborne Infectious Disease Outbreak Control (WIDOC) working group of the Water Environment Federation (Maal-Bared et al., 2020).
Kari Fitzmorris Brisolara, Rasha Maal-Bared, Robert S. Reimers, Albert Rubin, Mark D. Sobsey, Robert K. Bastian, Charles Gerba, James E. Smith, Kyle Bibby, Greg Kester, Sally Brown
Executive Summary
There have been concerns and studies related to potential effects to public health associated with municipal sludge. The development of USEPA 40 Code of Federal Regulations Part 503 was intended to ensure safety to the public and environment from infectious diseases (Reimers et al., 2004; USEPA, 1992). The regulations have been monitored and assessed by two reports from the National Academies of Sciences National Research Council (NRC, 1996; NRC, 2002). As indicated by these reports and further literature review, no direct disease-related effect has been established for Class A or Class B stabilized biosolids since the inception of these regulations. Because the COVID-19 virus is more susceptible to treatment (Figure 1), including heat, no additional protective equipment or measures are required for managing properly treated biosolids.
Figure 1. Pathogenic microorganisms present in wastewater sludge by pathogen class and their susceptibility to disinfection and environmental stress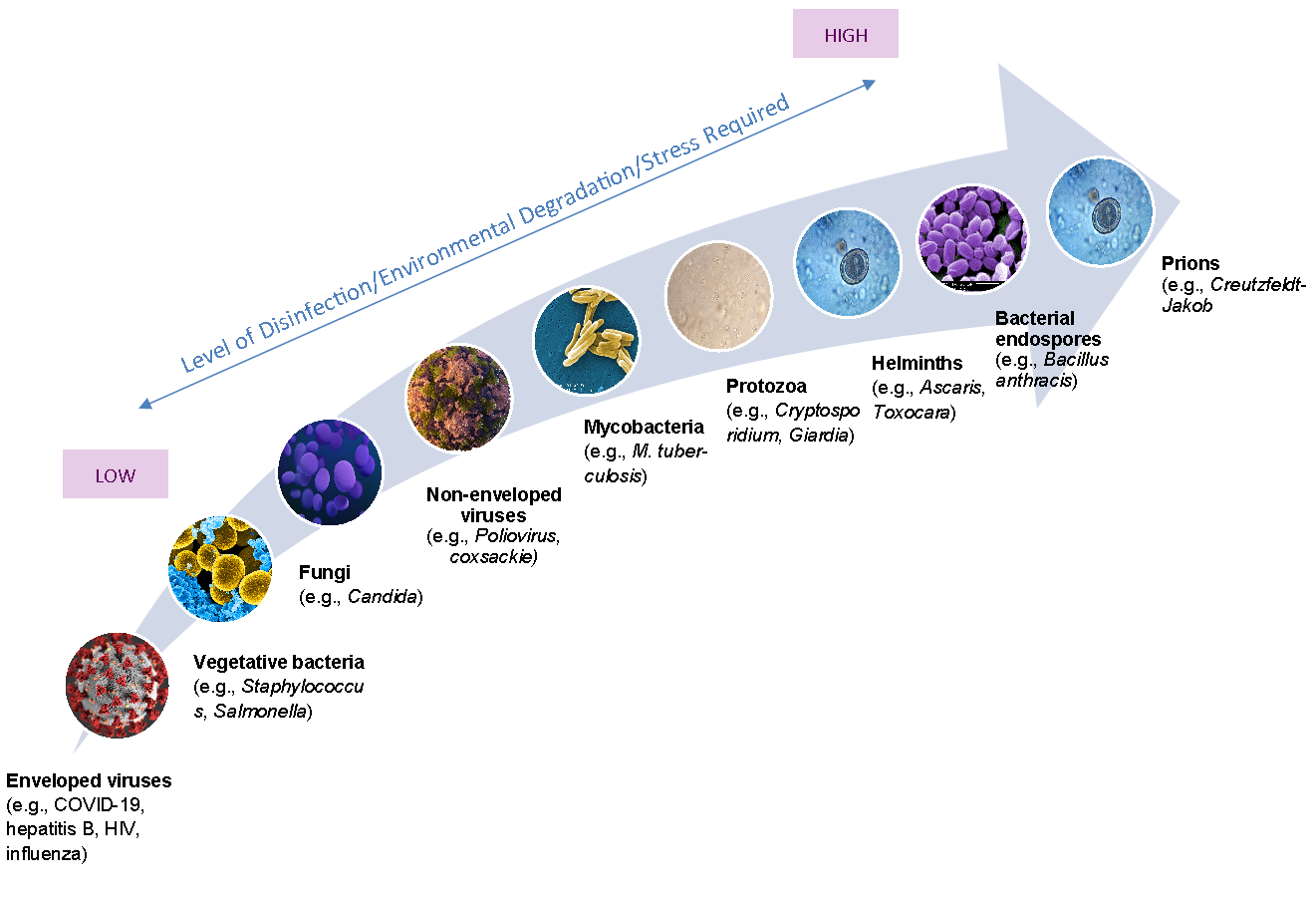
(EPA 1999; CDC 2008; Gattie & Lewis, 2004; Image: CDC Public Health Image Library
https://phil.cdc.gov/Default.aspx)
It should be emphasized that biosolids would not be expected to contain infectious COVID-19 virus. Currently, there is no documented evidence that biosolids (either Class A or B) are a source of transmission of coronavirus. The evidence indicates the main virus sources are respiratory droplets from infected people and respiratory deposits of these people on surfaces in direct contact with others —— that is, direct human-to-human contact.
Aerosol transmission also has not been clearly documented, except possibly for aerosol generation procedures in healthcare settings, such as respiratory therapy, used on severely ill patients as a part of their treatment and care (full infection control recommendations may be found at the U.S. Centers for Disease Control and Prevention (CDC) website under Precautions When Performing Aerosol Generating Procedures).
Contents
- Executive Summary
- Overview of Virus Density in Feces, Sludge and Biosolids
- Biosolids Disinfection Processes: Evaluation of Effectiveness and Regulation
- Presence of COVID-19 Virus in Each Level of Residuals and Likelihood of Transmission
- Recommendations
- Conclusions
- Authors
Overview of Virus Density in Feces, Untreated Sludge, and Biosolids
Residuals can be categorized into: (1) human feces, (2) untreated municipal sludge, (3) Class B biosolids and (4) Class A biosolids. Based on available literature, virus densities vary greatly by pathogen and by residual category and by process used for pathogen removal (see Table 1). Further discussions of viral load along with associated indicators such as bacteriophage was quantified in a review by Martin-Diaz et al. (2020). As viruses are typically adsorbed to solid particles both under natural and manmade conditions, certain solids (Fongaro et al. 2017; Hurst et al., 1980; Sobsey et al., 1980) and water characteristics can affect the adsorption efficiencies of viruses. These characteristics include pH, virus ionic characteristics (surface charge), virus surface hydrophobicity, dissolved salts, and organic matter among others (Hurst et al., 1980; Zhao et al., 2008; Bitton and Mitchell, 1974; Bixby; O’Brien, 1979). It has also been noted that other enveloped viruses used as COVID-19 virus surrogates (e.g. murine hepatitis virus (MHV) and Pseudomonas phage ϕ6) exhibit higher partitioning to solids compared to non-enveloped viruses (Ye et al., 2016).
Table 1. Summary of Virus Levels in Human Waste Residuals
|
Source |
Comments relevant to virus removal |
Average number of viruses/g matrix |
References |
|
Human Feces (Feachem et al., 1980; Shuval and Fattal, 2003; Metcalf and Eddy, 2014) |
It can be called Night Soil, Septage or Human Feces; without treatment, the infectivity of most viruses present in feces decreases by half within the range of 7 days to 6 months (Reimers et al. 2001; Madeley, 1979) |
Enteroviruses: 104 to 107
Hep A virus: 106
SARS: 106.1
|
(Shuval and Fattal, 2003)
(Shuval and Fattal, 2003)
(Hung et al., 2004) |
|
Untreated Municipal Sludge (Pederson, 1981)
|
WRRF report identified more than 250 viruses (Pillai et al., 2011). Infectivity ranged from 2 days to 6 months (Reimers et al., 2001). |
Coliphages: 102 – 107
Enteroviruses: 102 – 104
Noroviruses: 104 – 107
|
(Metcalf and Eddy, 2014; Yates et al., 2006) (Shuval andFattal, 2003; Reimers et al, 2001) (Gerba et al., 2011) |
|
Class B Biosolids (PSRP) (USEPA, 1992) |
Viruses found in partially disinfected biosolids will inactivate once applied to soil in outdoor field conditions between 30 days and 3 months |
Coliphages: 105 - 106
Enteroviruses: 102 - 103 |
(Yates et al., 2006)
(Shuval and Fattal, 2003)
|
|
Class A Biosolids (PFRP) (USEPA, 1992) |
Class A processes are required to obtain virus reduction to reach less than 1 PFU/4 grams dry weight solids. |
Enteroviruses: ≤0.25 (as determined by the detection limits of the assay) |
(Reimers et al., 2001) |
The extent to which COVID-19 virus is present in wastewater is unknown, and its presence in an infectious form has not been documented. The resulting illness has been shown, from a public health standpoint, to have a higher infectivity rate, but a lower case fatality rate than other coronaviruses previously studied including SARS-CoV-1 and MERS-CoV (NIAID-NIH, 2020; Ceccarelli et al., 2020; Pederson & Ho, 2020).
Recent evidence has highlighted the COVID-19 virus’s ability to attach to ACE2 receptors in the intestinal tract; details regarding infectivity of the COVID-19 virus may be found in the following references and also detailed in the WIDOC recommendations (Maal-Bared et al., 2020; Prussin et al., 2020; Rodriguez-Lazaro et al., 2012; Wigginton et al., 2012).
Biosolids Disinfection Processes: Evaluation of Effectiveness and Regulation
The disinfection processes included in approved biosolids treatments have been documented to inactivate pathogens more resistant to treatment than COVID-19 virus or any other enveloped viruses (AAMI, 2010; Gattie & Lewis, 2004; Wang et al. 2005; Wolff et al., 2005).
Members of the coronavirus family die off rapidly in wastewater, with the time required for the virus amounts to decrease 99.9% between 2 and 4 days before any treatment at 23°C (Gundy et al. 2009). Even in the examination of enteric viruses, typical wastewater treatment mechanisms (primary sedimentation, trickling filter/activated sludge, disinfection or coagulation, filtration, disinfection) have been shown to achieve a greater than 99.9% reduction in viral load (Pepper et al., 2006).
Mesophilic anaerobic digestion reduced enteric virus (believed to be largely enteroviruses) numbers in digested sludge by an average of 94.4% (1.97 log10), while thermophilic anaerobic digestion reduced enteric viruses to below detection limits (less than 316 virus/mL) (Sassi et al., 2017). Wang et al. (2005) noted SARS Cov-1 virus was more susceptible to disinfectants (free chlorine more effective than chlorine dioxide) than E. coli and f2 phage. Table 2 highlights the ranges in log reductions by sludge treatment type (Godfree, 2003). Coronaviruses have been shown to survive up to 9 days on surfaces; inactivation can be achieved with 62% to 71% ethanol, 0.5% hydrogen peroxide, or 0.1% sodium hypochlorite within 1 minute with higher temperatures reducing survival (Kampf et al., 2020). These disinfectants can be helpful when addressing concerns related to potential surface contamination that workers may come into contact with in the WRRF.
Table 2. Summary of pathogen reductions during sludge treatment (Godfree et al., 2003; Ward et al., 1984)
|
|
Log10 reduction range |
||
|
Treatment |
Bacteria |
Viruses |
Parasites |
|
Mesophilic anaerobic digestion |
0.5 to 4 |
0.5 to 2 |
0 |
|
Aerobic digestion |
0.5 to 4 |
0.5 to 2 |
0 |
|
Composting |
2 to >4 |
2 to >4 |
2 to >4 |
|
Air drying |
0.5 to 4 |
0.5 to >4 |
0.5 to >4 |
|
Lime stabilization |
2 to >4 |
>4 |
0 |
When it comes to biosolids treatment, for a process to achieve equivalency status, it must be approved by the EPA’s Pathogen Equivalency Committee (PEC) (Fitzmorris et al., 2007). Table 3 broadly shows EPA’s requirements for demonstrating equivalency of an innovative or alternative technology to a PSRP or PFRP equivalent process. PEC has a strict protocol for this evaluation that includes describing the exact mechanism of disinfection along with key process control parameters. To demonstrate the required log reductions, the untreated sludge must contain an adequate number of organisms. For example, to demonstrate PFRP equivalency untreated sludge must contain more than 1,000 PFU of enteric viruses /4 g TS (dry weight basis); and 100 viable Ascaris spp. ova/4 g TS. If the untreated sludge does not naturally contain these density levels, the applicant must spike the sample to demonstrate the process’ capability for achieving a 3-log reduction in enteric viruses and a 2 log reduction in viable Ascaris spp. ova.
Table 3. Requirements for Demonstrating Equivalency*
|
PSRP Equivalency |
PFRP Equivalency |
|
> 1 log reduction of Salmonella sp. or > 2 log reduction of fecal coliforms |
> 3 log reduction of enteroviruses |
|
> 1 log reduction of enteroviruses |
> 2 log reduction of viable Ascaris sp. ova |
|
Final product contains < 2,000,000 fecal coliforms/g |
Final product contains < 1000 fecal coliforms or < 3 Salmonella sp./4 g; < 1 pfu/4g of enteroviruses and < 1 helminth ova/ 4g |
*Detailed information on demonstrating equivalency can be found at https://www.epa.gov/biosolids/pathogen-equivalency-committee.
Most virus-related research in the biosolids field has been related to enteroviruses. Work by Gundy et al. (2009) showed that SARS-CoV-1, a relative of the COVID-19 virus, displays less environmental persistence in primary and secondary effluent compared to poliovirus, which is used as an indicator for biosolids PFRP equivalency. Wang et al. (2005) noted SARS Cov-1 virus was more susceptible to disinfectants (free chlorine more effective than chlorine dioxide) than E. coli and f2 phage. Recent work has observed that reoviruses and bacteriophages could be excellent surrogate indicators for viruses due to their resistance to treatment (Diaz et al., 2020; Viau and Peccia, 2009; Gerba et al., 2018). It should be noted that proposed surrogates that are non-enveloped viruses are expected to display higher levels of resistance to environmental stressors and disinfection compared to enveloped viruses in general and COVID-19 virus specifically, thereby making them conservative indicators (CDC, 2020).
In non-centralized treatment such as septic tanks, previous research related to Ebola from 2015 indicated current World Health Organization guidance of holding latrine waste for longer than 7 days was sufficient to inactivate enveloped viruses (Casanova, 2015). This data represents pit latrine conditions, not current septic tanks or decentralized treatment facilities currently in place in the U.S. Therefore, these recommendations are particularly protective if treatment is via a current septic tank or decentralized treatment facility.
Presence of COVID-19 Virus in Each Level of Residuals and Likelihood of Transmission
A traditional risk assessment includes four stages: a) hazard identification; b) exposure assessment; c) dose–response assessment, and d) risk characterization. As the infective dose of COVID-19 virus remains unknown, performing a quantitative microbial risk assessment is not possible. We can, however, perform a qualitative risk assessment that is based on a hazard assessment — that is, presence and infectivity in all four categories of residuals and on an exposure assessment that examines likelihood of transmission.
Presence:
Presence of SARS Cov-1 RNA in stool samples was noted by He et al. (2004) in 57.4% of SARS patients (FQ-PCR method, infectivity not determined). Additional quantification of median duration of SARS Cov-1 virus excretion from stools was 27 days (16 to 126 day range) but no infectivity was noted (Liu et al. 2004). Studies on other coronaviruses, namely SARS Cov-1 indicated the virus only survived in in-vitro experiments 3 days in feces, but at 4°C persisted for 17 days in feces (Wang et al., 2005). Detection frequencies of the genetic material (RNA) of other coronavirus in feces of infected individuals ranged from 16% to 97% with a peak for SARS-CoV-1 RNA at 9 to 14 days after illness onset (Cheng et al., 2004; Wigginton et al., 2015). Three other human coronaviruses — HCoVNL63, HCoV-OC43, and HCoV-229E — that infect the upper respiratory tract have been detected in stool samples (Esper et al., 2010; Risku et al., 2010).
Excerpt from Maal-Bared et al. (2020):
While there are limited data on the concentrations of enveloped viruses in feces and urine (Wigginton and Boehm, 2020), various reports have now confirmed the presence of COVID-19 virus RNA in stool samples from positive patients (Wu et al., 2020; Chen et al., 2020; Holshue et al., 2020; Xiao et al., 2020; Ong et al., 2020; Tang et al., 2020b; Woelfel et al., 2020). Wang et al. (2020) reported the percentage of RNA positive fecal samples in a sample of 205 patients to be 29%. Chen et al. (2020) reported 64% of fecal samples to be RNA positive in a sample of 44 patients. Wölfel et al. (2020) reported that stool samples remained RNA-positive over three weeks in six of the nine examined patients, in spite of full resolution of symptoms. Wölfel et al. (2020) also remarked that no infective virus was detected in spite of high RNA concentrations in feces. The authors recommended that future work evaluate if exposure to the gut environment inactivates COVID-19 virus. To this date, only two studies (Zhang et al. 2020a; Wang et al 2020) were able to detect infective virus in feces. These studies confirmed intact viral presence with electron microscopy but did not quantify the viral particles detected. The latter suggested that the detection of infective virus is highly dependent on virus concentrations in feces (Wang et al., 2020).
Likelihood of transmission through exposure:
The main mode of transmission of COVID-19 virus is person-to-person contact through respiratory droplets produced when an infected person coughs, sneezes, or talks. These droplets can land in the mouths or noses of people who are nearby or possibly be inhaled into the lungs. Evidence from other coronaviruses shows that these viruses can survive on surfaces (i.e., fomites), especially when surrounded by mucous for up to 9 days (Kampf, 2020). Similar studies have been conducted for COVID-19 virus (van Dormalen et al. 2020; Chin et al., 2020). Thus, cleaning of PPE, shared equipment, and frequently touched surfaces is advisable to interrupt this possible route of transmission.
There is currently little epidemiological or virological evidence that COVID-19 virus is transmitted by the fecal–oral route. The only documented incident occurred for the SARS coronavirus in Hong Kong, where possible evidence of airborne exposure was suggested as the source of transmission due to faulty wastewater piping in a building (Brown, 2020). Both the World Health Organization (WHO) and further studies have indicated airborne transmission was likely, but did not present evidence linking the airborne transmission to feces (WHO, 2003; Yu et al., 2014; Yu et al., 2004). Additionally, recent work has shown that COVID-19 virus binds to the angiotensin-converting enzyme 2 (ACE2) receptors on Type II alveolar cells and intestinal epithelia (Hoffman et al., 2020; Wan et al., 2020; Wrapp et al., 2020). Its ability to attach to these cell receptors in the intestinal tract supports the potential for fecal–oral transmission. Based on the limited number of studies that were able to culture viable COVID-19 virus from fresh feces, it is reasonable to assume that the highest risk of infection would be related to exposure to freshly excreted feces in the bathroom, plumbing system, and the collections system (Gormley et al., 2020) and in conditions where recommended hygiene practices are difficult to apply (Lodder and de Roda Husman, 2020).
Presence:
From the Netherlands, Australia, and the U.S., COVID-19 virus genetic material (nucleic acid) but not infectious virus were found in municipal wastewater (Medema et al., 2020; Ahmed et al., 2020; Wu et al., 2020). They noted the occurrence of coronavirus genes in municipal wastewater before cases were identified (Medema et al., 2020). Previous studies also have noted untreated wastewater sludge and Class B biosolids contained genetic material from other coronaviruses in more than 80% of the samples (as were Klassevirus and Rotavirus) (Bibby and Peccia, 2013). Again, this assessment was performed by nucleic acid (genetic) analysis and does not indicate infectivity of the viruses.
Likelihood of transmission through exposure:
Current practices related to the appropriate use of personal protective equipment (PPE) when in potential contact with untreated wastewater and municipal sludge are considered to be effective, even in the vicinity of processes that create airborne droplets and aerosols. The Water Environment Federation has convened a Blue-Ribbon Panel to evaluate biological hazards and precautions for wastewater workers – there will be more information forthcoming from this group. In general, agencies should follow recommendations from the U.S. Occupational Safety and Health Administration (OSHA) and local occupational health and safety agencies based on the conditions at their local facilities.
Presence:
Since the coronaviruses are fragile and typically at a very low density in wastewater, the likelihood that Class B biosolids will contain infective COVID-19 virus is low (Wolff et al., 2005). Concentrations in Class B biosolids of enteric viruses, which are more resistant to treatment than COVID-19 virus, after mesophilic anaerobic digestion ranged between 101 and 104 MPN/gram (Wong et al., 2010).
Likelihood of transmission through exposure:
Potential risk from pathogenic microorganisms associated with land application of biosolids vary based on the biosolids application method, the microbiological quality of the biosolids at the time of application, environmental factors, and the management of the site (Yates et al., 2006). Thus, when evaluating the risk of COVID-19 virus transmission from biosolids the assessment must include the nature of the residuals in question. In the highly unlikely probability that a viable COVID-19 virus is present in Class B biosolids, models of bioaerosol transport indicate little risk to surrounding communities and workers at application sites. In fact, no risk was determined from aerosolized viruses at estimated distances of 10,000 m from the site and a 3% risk to workers on site (2 m/s wind, 1 hour of exposure) (Dowd et al., 2000). It is also of note that these models tend to overestimate actual risk of populations by the nature of their design. Indeed, though application methods vary, there is no indication of viruses found further than 5 m from application when they were even found in the aerosols at all (Brooks et al., 2004).
Westrell et al. (2004) examined the application of hazard analysis and critical control points along with quantitative microbial risk assessment tools to quantify risk to workers and communities surrounding land application sites (Class B, mesophilic anaerobically digested biosolids). They found the highest individual risk from a single exposure occurred via aerosols for workers at the belt press for dewatering (prior to Class B treatment) and overall risk was highest in the early processes, prior to stressors/disinfection as opposed to during land application (Westrell et al., 2004). While there is concern, it is highly unlikely infection can occur from Class B biosolids based on current evidence (Sobsey, 2020). Over the last 40 years, there have not been recorded infections due to viruses in Class B biosolids applications as noted by the National Research Council reports (NRC, 1996 and 2002). No additional measures are needed for protecting workers against COVID-19 virus beyond what is typically used when handling Class B biosolids. See CDC recommendations for additional details on these measures.
Presence:
It is of note that the vast majority of research related to virus presence and survival in biosolids, including research on the effectiveness of treatment, has been focused on non-enveloped viruses. Non-enveloped viruses, such as Poliovirus, are much more robust than enveloped viruses, such as the COVID-19 virus.
One study on these non-enveloped, primarily enteric viruses showed thermophilic digestion to be more effective than mesophilic anaerobic digestion (Wong et al., 2010). Mesophilic anaerobically digested Class B biosolids further treated to Class A with heat pelletization (35° to 37°C for 10 to 20 days, dewatering, followed by a low-pressure oxidation drying system) and composting (agitated windrow method) resulted in even lower virus levels (Viau and Peccia 2009).
Likelihood of transmission through exposure:
The risks of contracting COVID-19 from Class A biosolids are negligible. The virus itself is less stable than the virus surrogate utilized to get approval as disinfected Class A biosolids by the EPA PEC (Wang et al., 2005).
Reccomendations
In alignment with CDC and OSHA recommendations, we recommend that workers and employers manage untreated residuals and sludge with potential or known COVID-19 virus contamination like any other untreated material. As noted by LeChevallier et al. (2019) there is a hierarchy of PPE that follows the treatment through the WRRF. As material progresses through treatment, risk decreases. Contact transfer PPE including gloves, boots, and uniform/coveralls should be utilized throughout WRRF operations, with the addition of safety glasses/goggles or face shields in areas with splash hazards. Use of typical engineering and administrative controls, safe work practices, and personal protective equipment (PPE) when handling untreated wastewater, feces, and untreated municipal sludge to prevent worker exposure is important.
Figure 2. How to Stay COVID-19 Free at the WRRF

In general, agencies should follow recommendations from OSHA and the state agency responsible for occupational health and safety. More specifically, the following recommendations should be highlighted during this time (Rubin, 2020; Burton and Trout, 2009):
- As is current practice, remain mindful of the operation; take precautions to minimize the production of aerosols from feces and municipal sludge in the collections system and in the headworks of the WRRF. In addition, when land applying Class B biosolids, observe site buffer requirements as outlined in permits.
- Management notes: Periodic training regarding standard hygiene practices and PPE use and maintenance should continue. Ensure workers are up to date on CDC-recommended vaccinations such as tetanus–diphtheria and hepatitis A immunizations.
- Management notes: Periodic training regarding standard hygiene practices and PPE use and maintenance should continue. Ensure workers are up to date on CDC-recommended vaccinations such as tetanus–diphtheria and hepatitis A immunizations.
- Wash hands often, carry disinfecting wipes and disinfecting gel to use anytime the operator touches surfaces that may have been exposed to aerosols. Switches, valves, and other surfaces exposed to aerosol from processes prior to complete biosolids treatment should be sanitized. Operators also should sanitize exposed surfaces prior to leaving an area to assure surfaces are as sanitary as possible and do not pose a hazard to other personnel.
- Management notes: Hand-washing stations with clean water and mild soap should be readily available wherever contact with wastewater or sludge may occur.
- Management notes: Hand-washing stations with clean water and mild soap should be readily available wherever contact with wastewater or sludge may occur.
- During operation, wear heavy rubber gloves over surgical gloves as well as goggles or face shield. If surgical gloves are used, discard them and sanitize the outer rubber gloves.
- Management notes: CDC has established guidelines for workers along with WHO.
- Management notes: CDC has established guidelines for workers along with WHO.
- At the end of a shift at the office or maintenance shop, change out field clothes, place in clothes hamper for dirty clothes/uniforms/shower, and change into street clothes before heading home. If your go directly from the field to home, take steps to protect family members — remove clothes in mudroom, garage, or laundry room. Handle as dirty clothes do not commingle dirty clothes from the household with field clothes. Remove field boots before entering home and wash boots (especially the bottoms) before entering house, office, or personal vehicle.
- In summary, be aware of hazards. Wash hands frequently — before smoking, before drinking water or other drinks, before eating. Avoid hand contact with face and do not touch face with glove covered hands. Face coverings can help with protecting face from contact with gloves.
Conclusions
Based on evidence presented, there is no currently available epidemiological data that establishes a direct link between wastewater sludge or biosolids and risk of infection from the COVID-19 virus. Despite shedding of the virus RNA in feces, there is no evidence supporting the transmission of COVID-19 virus through the wastewater system including biosolids. Research efforts have been undertaken to quantify the presence of the virus in wastewater, but infectivity thus far has not been included in these assessments. Put simply, presence of RNA in wastewater does not indicate the virus is necessarily infective. Additional research is needed to directly assess the public health risks of the COVID-19 infection via feces and untreated sludge.
Even though there is no evidence establishing the presence of infectious COVID-19 virus in feces, wastewater, or biosolids, workers should remain vigilant and practice good hygiene and effective safety practices to minimize the risks of exposure to any viruses or other pathogens from these potential sources.
Authors
- Kari Fitzmorris Brisolara is the Associate Dean for Academic Affairs and an Associate Professor of Environmental and Occupational Health Sciences at the Louisiana State University Health Sciences Center (New Orleans, Louisiana) and Vice Chair of the WEF Disinfection and Public Health Committee.
- Rasha Maal-Bared is the Senior Microbiologist at EPCOR Water Services Inc. (Edmonton, Canada) and the current chair of the Waterborne Infection Disease Outbreak Control Working Group.
- Robert S. Reimers is a Professor Emeritus at Tulane University’s School of Public Health and Tropical Medicine and Director of Environmental Solutions, Pinnacle Waste Solutions, LLC (Richmond, Texas).
- Albert Rubin is a professor emeritus at North Carolina State University in the Dept of Biological and Agricultural Engineering.
- Mark D. Sobsey was the Kenan Distinguished Professor of Environmental Sciences and Engineering at the Gillings School of Global Public Health at the University of North Carolina (Chapel Hill). He has served as an advisor on numerous national and international drinking water and WASH committees for the World Health Organization, WEF, and AWWA.
- Robert Bastian is a is a 2016 Water Environment Federation Fellow.
- Charles Gerba is a professor of epidemiology and biostatistics in the Dept of Environmental Science at the University of Arizona and a supporting member of the WIDOC working group.
- James E. Smith is a retired senior environmental engineer.
- Kyle Bibby is an Associate Professor and the Wanzek Collegiate Chair in the Department of Civil and Environmental Engineering and Earth Sciences at the University of Notre Dame (Notre Dame, Indiana).
- Greg Kester is Director of Renewable Resources Program, California Association of Sanitation Agencies (Sacramento, California).
- Sally Brown is a research professor at the University of Washington (Seattle).
•••
Monitor WEF’s coronavirus resource page for the most up-to-date information and resources for the water sector.
Download a PDF of this article:
Residuals and Biosolids Issues
Concerning COVID-19 Virus
(WSEC-2020-TR-001)
•••
Notable Updates:
Since the date of publication of this article, notable new and updated research has emerged. You can view these notable updates here.
•••
Listen to author Kari Fitzmorris Brisolara discuss this article on the WEF Words on Water podcast.
References
Advancing Safety in Medical Technology (AAMI). 2010. Designing, testing, and labeling reusable medical devices for reprocessing in health care facilities: A guide for medical device manufacturers. AAMI TIR12:2010 (Revision of AAMI TIR12:2004).
Barber, G., 2020. “One Way to be Potentially Track Corid-19?” Sewage Surveillance Science.
Babatola, A. and Reimers, R.S., Developed and Co-Chaired WEFTEC Workshop on Bacteriophage Analyses for Biosolids and Water Quality Standards Assessment, Chicago, Illinois (September 2017)
Bibby, K. and Peccia, J. 2013. “Identification of Viral Pathogen Diversity in Sewage Sludge by Metagenome Analysis.” Environ. Sci. Technol. 47(4): 1945-1951.
Bixby, R. and O’BrienD. 1979. “Influence of fulvic acid on bacteriophage adsorption and complexation in soil.” Appl. Environ. Microbiol., 38(5), 840-845.
Brooks, J., Gerba, C., and Pepper, I. 2004. “Aerosol Emission, Fate, and Transport from Municipal and Animal Wastes.” J. Residuals Sci. Technol. 1(1), 13-25.
Brown, S. 2020. “COVID-19: A Biosolids Science Perspective, BioCycle the Organics Recycling Authority,” www.biocycle.act/2020/03/20/covid-19-science -perspective
Burton, N. C., Trout, D. 1999. Health Hazard Evaluation Report 98–0118–2748 Bio–Solids Land Application Process LeSourdsville, NIOSH, Ohio.
Casanova, L. 2015. “Inactivation of an Enveloped Surrogate Virus in Human Sewage.” Environ. Sci. Technol. Lett. 2015, 2, 76−78. DOI: 10.1021/acs.estlett.5b00029
Ceccarelli, M., Berretta, M., Rullo, E., Nunnari, G., Cacopardo, B. 2020. “Editorial – Differences and similarities between Severe Acute Respiratory Syndrome (SARS)-CoronaVirus (CoV) and SARS-CoV-2.” European Review Med. Pharmacol. Sci. 24, 2781-2783.
Centers for Disease Control (CDC). 2008. Guideline for Disinfection and Sterilization in Healthcare Facilities. https://www.cdc.gov/infectioncontrol/guidelines/disinfection/; last update May 2019.
Cheng, P., Wong, D., Tong, L., Ip, S., Lo, C., Lau, C., Yeung, E, and Lim, W. 2004. “Viral shedding patterns of coronavirus in patients with probable severe acute respiratory syndrome.” The Lancet, 363, 1699–1700.
Diaz, J., Lucena, F., Blanch, A., Jofre, J. 2020. “Review: Indicator bacteriophages in sludge, biosolids, sediments and soils.” Environ. Res. 182, https://doi.org/10.1016/j.envres.2020.109133
Dowd, S., Gerba, C., Pepper, I., and Pillai, S. 2000. “Bioaerosol Transport Modeling and Risk Assessment in Relation to Biosolid Placement.” J. Environ. Qual. 29(1), 343-348. https://doi.org/10.2134/jeq2000.00472425002900010043x
Esper, F., Ou, Z., Huang, Y. 2010. “Human coronaviruses are uncommon in patients with gastrointestinal illness.” J. Clin. Virol. 48(2):131-133. DOI: 10.1016/j.jcv.2010.03.007
Feachem, R,G., Bradeu. D.J., Garelick, H. and Mara, D.D., 1980. Health Aspects of Excreta and Sullage Management - A State of the Art Review, The World Bank, Washington, DC
Fitzmorris, K.B., Reimers, R.S., Pillai, S.D., Pillai, S.D., Oleszkiewicz, J.A. and Smith, J.E., 2007. “Production of Safe Biosolids from Agricultural and Municipal Residuals’ Emerging Physical Chemical Processes,” International Water Association Specialty Conference Proceedings Moving Forward Wastewater Biosolids Sustainability: Technical, Management and Public Synergy, International Water Association, Greater Moncton Sewerage Commission, Moncton, New Brunswick, Canada. Pp 1069-1076.
Fitzmorris, K.B. and Reimers, R.S., 2009. “Assessment of Indicator Organisms and Long-Term Stability in Advanced Alkaline Systems,” WEF Disinfection 2009 Specialty Conference, Atlanta, Georgia, CD-ROM.
Gattie, D., Lewis, D. 2004. “A High-Level Disinfection Standard for Land-Applied Sewage Sludges.” Environ. Health Perspect. 112(2). https://doi.org/10.1289/ehp.6207
Gerba, C., Betancourt, W., Kitajima, M., Rock, C. 2018. “Reducing uncertainty in estimating virus reduction by advanced water treatment processes.” Water Res. 133(15), 282-288. https://doi.org/10.1016/j.watres.2018.01.044
Gerba, C., Tamimi, A., Pettigrew, C., Weisbrod, A., Rajagopalan, V. 2011. ”Sources of microbial pathogens in municipal solid waste landfills in the United States of America.” Waste Manage. Res. 29(8), 781-790. DOI: 10.1177/0734242X10397968
Godfree, A. 2003. “Health constraints on the agricultural recycling of wastewater sludges.” Mara, D., & Horan, N. J. (Eds.). (2003). Handbook of Water And Wastewater Microbiology. Elsevier, London, UK.
Gormley, M., Aspray, T., Kelly, D. 2020. “COVID-19: mitigating transmission via wastewater plumbing systems.” The Lancet, DOI: https://doi.org/10.1016/S2214-109X(20)30112-1
Gundy, P., Gerba, C., Pepper, I. 2009. ”Survival of Coronaviruses in Water and Wastewater.” Food Environ. Virol. 1, 10-14. DOI 10.1007/s12560-008-9001-6
He, Z., Dong, Q., Song, S., He, L., Zhuang, H. 2004. “Detection for severe acute respiratory syndrome (SARS) coronavirus RNA in stool of SARS patients.” Chinese J. Prevent. Med. 38(2), 90-91.
Hoffmann, M., Kleine-Weber, H., Krüger, N., Mueller, M. A., Drosten, C., & Pöhlmann, S. (2020). “The novel coronavirus 2019 (2019-nCoV) uses the SARS-coronavirus receptor ACE2 and the cellular protease TMPRSS2 for entry into target cells.” BioRxiv. https://www.biorxiv.org/content/10.1101/2020.01.31.929042v1.abstract
Holshue, M.L., et al. “First Case of 2019 Novel Coronavirus in the United States.” New England Journal of Medicine (2020). DOI: 10.1056/NEJMoa2001191.
Hung, I. F. N., Cheng, V. C. C., Wu, A. K. L., Tang, B. S. F., Chan, K. H., Chu, C. M., ... & Chan, K. S. (2004). “Viral loads in clinical specimens and SARS manifestations.” Emerging infectious diseases, 10(9), 1550.
Kampf, G., Todt, D., Pfaender, S., Steinmann, E. 2020. “Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents.” J. Hosp. Infection. 104, 246-251.
Liu, W., Tang, F., Fontanet, A., Zhan, L., Zhao, Q., Zhang, P., et al. 2004. “Long-term SARS Coronavirus Excretion from Patient Cohort,” China. Emerg. Infect. Dis. 10(10), 1842-1843. doi: 10.3201/eid1010.040297
Lodder, W., de Roda Husman A.M. (2020). “SARS-CoV-2 in wastewater: potential health risk, but also data source.” The Lancet Gastroenterology and Hepatology. DOI: https://doi.org/10.1016/S2468-1253(20)30087-X
Madeley, C. 1979. “Viruses in the stools.” J. Clin. Pathol. 32, 1-10.
Medema, G., Heijnen, L., Elsinga, C. and Ltlegander, R., 2020. “Presence of SAR-Coronavirus-2 in Sewage” from KWR Water Research Institute in Nieuwegein, Netherlands.
Metcalf & Eddy, Abu-Orf, M., Bowden, G., Burton, F. L., Pfrang, W., Stensel, H. D., ... & AECOM (Firm). (2014). Wastewater Engineering: Treatment and Resource Recovery. McGraw Hill Education.
National Institute of Allergy and Infectious Disease (NIAID), National Institute of Health (NIH). 2020. COVID-19, MERS & SARS. https://www.niaid.nih.gov/diseases-conditions/covid-19
National Research Council (NRC), 1996. Use of Reclaimed Water and Sludge in Food Crop Production, National Academy Press, Washington, DC
National Research Council (NRC), 2002. Biosolids Applied to Land – Advincing Standards and Practices, National Academy Press, Washington, D.C.
Ong, S. et al. (2020). “Air, Surface Environmental, and Personal Protective Equipment Contamination by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) From a Symptomatic Patient.” JAMA.
Pederson, D.C., 1981. Density Levels of Pathogenic Organisms in Municipal Wastewater Sludge – A Literature Review. EPA-6001S2-81-170, Cincinnati, Ohio
Pederson, S., Ho, Y. 2020. “SARS-CoV-2: a storm is raging.” J. Clin. Investigation. https://doi.org/10.1172/JCI137647
Pepper, I., Brooks, J., Gerba, C. 2006. “Pathogens in Biosolids.” Adv. Agronomy, 90, 1-41. https://doi.org/10.1016/S0065-2113(06)90001-7
Pillai, S.D., Meckes, M.C., Murthy, S.N. and Willis, J., 2011. Developing Better, Indicators for Pathogen Presence in Sewage, Sludge. Report to Water Environmental Research Foundation (WERF), Washington, D.C.
Pratt, L.S., Reimers, R.S., Jeng, H.W., Bowman, D.D., Oleszkiewicz, J.A. Meckes, M. and Fitzmorris, K.B., 2005. “Development of surrogate indicators to monitor pathogens in biosolids,” Joint Residuals and Biosolids Management Conference 2005 – Advancing the State of Technology, Water Environment Federation, Alexandria, VA, CD-ROM.
Reimers, R.S., Bowman, D.B., Schafer, P.L. Tata, P. Leftwich, B. and Atique, M.M., 2001. “Factors Affecting Lagoon Storage Disinfection of Biosolids,” WEF Residuals and Biosolids Specialty Conference, Water Environment Federation, Alexandria, VA, CD-ROM
Reimers, R.S., Fitzmorris, K.B., Smith, J.E., Boyd, G.R., and Bowman, D. D., 2004. “State of Art in Treatment and Survival of Pathogens in Biosolids,” Journal of Residuals Science and Technology, Volume 1, Number 2 , DEStech Publications Inc., Lancaster, Pennsylvania, pp. 93-104.
Risku, M., Lappalainen, S., Rasanen, S., Vesikari, T. 2010. “Detection of human coronaviruses in children with acute gastroenteritis.” J. Clin. Virol. 48(1):27-30. doi: 10.1016/j.jcv.2010.02.013.
Rubin, A.R., 2020, Personal Communication
Sassi, H., Ikner, L., Abd-Elmaksoud, S., Gerba, C., Pepper, I. 2018. “Comparative survival of viruses during thermophilic and mesophilic anaerobic digestion.” Sci. Tot. Environ. 615, 15-19.
Shuval, H., Fattal, B. 2003. “Control of pathogenic microorganisms in wastewater recycling and reuse in agriculture.” Mara, D., & Horan, N. J. (Eds.). (2003). Handbook of Water and Wastewater Microbiology. Elsevier, London, UK.
Sobsey, M., 2020. Personal Communication
Tang, A., Tong, Z. D., Wang, H. L., Dai, Y. X., Li, K. F., Liu, J. N., ... & Yan, J. B. (2020). “Detection of Novel Coronavirus by RT-PCR in Stool Specimen from Asymptomatic Child,” China. Emerging infectious diseases, 26(6).
USEPA, 1992 (revised 2003). Environmental Regulations and Technology – Control of Pathogens and Vector Attraction in Sewage Sludge, 40 CFR Part 503, EPA/625/R-92/013. Cincinnati, Ohio
van Doremalen, N., Bushmaker, T., Morris, D. H., Holbrook, M. G., Gamble, A., Williamson, B. N., ... & Lloyd-Smith, J. O. (2020). “Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1.” New England Journal of Medicine.
Viau, E. and Peccia, J. 2009. “Survey of Wastewater Indicators and Human Pathogen Genomes in Biosolids Produced by Class A and Class B Stabilization Treatments.” Appl. Environ. Microbiol. 75(1), 164-74.
Wan, Y., Shang, J., Graham, R., Baric, R. S., & Li, F. (2020). “Receptor recognition by the novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS coronavirus.” Journal of virology, 94(7). https://jvi.asm.org/content/94/7/e00127-20.abstract
Wang, X., Li, J., Jin, M., Zhen, B., Kong, Q., Song, N., et al. 2005. “Study on the resistance of severe acute respiratory syndrome-associated coronavirus.” J. Virol. Methods, 126(1), 171-177. doi: 10.1016/j.jviromet.2005.02.005
Wang, W., Xu, Y., Gao, R., Lu, R., Han, K., Wu, G., & Tan, W. (2020). “Detection of SARS-CoV-2 in Different Types of Clinical Specimens.” JAMA. https://jamanetwork.com/journals/jama/fullarticle/2762997
Westrell, T., Schönning, C., Stenström, T. A., & Ashbolt, N. J. (2004). “QMRA (quantitative microbial risk assessment) and HACCP (hazard analysis and critical control points) for management of pathogens in wastewater and sewage sludge treatment and reuse.” Water Sci. Technol., 50(2), 23-30.
Wigginton, K., Ye, Y., and Ellenberg, R. 2015. “Emerging investigators series: the source and fate of pandemic viruses in the urban water cycle.” Environ. Sci.: Water Res. Technol., 1, 735.
Wigginton, K. R., & Boehm, A. B. (2020). “Environmental Engineers and Scientists Have Important Roles to Play in Stemming Outbreaks and Pandemics Caused by Enveloped Viruses.” Environmental Science & Technology, 54 (7), 3736-3739. https://pubs.acs.org/doi/full/10.1021/acs.est.0c01476
Wölfel, R., Corman, V.M., Guggemos, W. et al. ”Virological assessment of hospitalized patients with COVID-2019.” Nature (2020). https://doi.org/10.1038/s41586-020-2196-x
Wolff, M., Sattar, S., Adegbunrin, O., and Tetro, J. 2005. “Environmental survival and microbicide inactivation of coronaviruses.”on Coronaviruses with Special Emphasis on First Insights Concerning SARS ed. Schmidtt, Wolff, Weber ISBN 3-7643-6462-9
Wong, K., Onan, B., and Xagoraraki, I. 2010. “Quantification of Enteric Viruses, Pathogen Indicators, and Salmonella Bacteria in Class B Anaerobically Digested Biosolids by Culture and Molecular Methods.” Appl. Environ. Microbiol., 76, 6441–6448.
World Health Organization (WHO). 2003. Severe Acute Respiratory Syndrome (SARS) - multi-country outbreak - Update 15. https://www.who.int/csr/don/2003_03_31/en/
Wrapp, D., Wang, N., Corbett, K. S., Goldsmith, J. A., Hsieh, C. L., Abiona, O., ... & McLellan, J. S. (2020). “Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation.” Science, 367(6483), 1260-1263.
Yates, M. V., & Yates, S. 2007. Assessing the Fate of Emerging Pathogens in Biosolids. Water Environment Research Foundation.
Ye, Y., Ellenberg, R., Graham, K., and Wigginton, K. 2016. “Survivability, Partitioning, and Recovery of Enveloped Viruses in Untreated Municipal Wastewater.” Environ. Sci. Technol. 50(10), 5077-5085. https://doi.org/10.1021/acs.est.6b00876
Yu, I., Li, Y., Wong, T., Tam, W., Chan, A., Lee, J., Leung, D., Ho, T. 2004. “Evidence of airborne transmission of the severe acute respiratory syndrome virus.” N. Engl. J. Med. 350(17):1731-9. DOI: 10.1056/NEJMoa032867
Yu, I., Qui, H., Tse, L., Wong, T. 2014. “Severe Acute Respiratory Syndrome Beyond Amoy Gardens: Completing the Incomplete Legacy.” Clinical Infectious Diseases, 58(5), 683-686. https://doi.org/10.1093/cid/cit797
Zhang, Y. et al. 2020. “Isolation of 2019-nCoV from a Stool Specimen of a Laboratory-Confirmed Case of the Coronavirus Disease 2019 (COVID-19)[J].” China CDC Weekly, 2020b, 2(8): 123-124.


